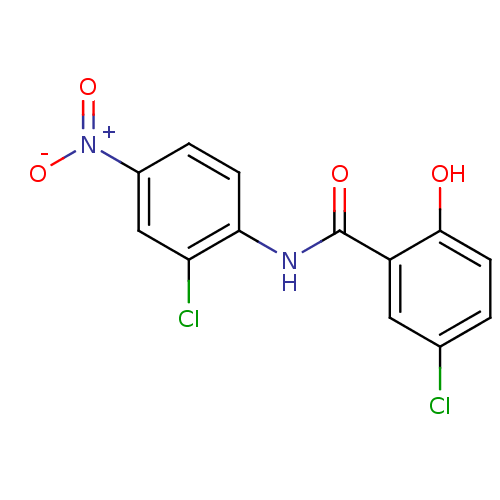
BDBM11242 5-chloro-N-(2-chloro-4-nitrophenyl)-2-hydroxybenzamide::med.21724, Compound 76::niclosamide
SMILES Oc1ccc(Cl)cc1C(=O)Nc1ccc(cc1Cl)[N+]([O-])=O
InChI Key InChIKey=RJMUSRYZPJIFPJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 11242
Found 3 hits for monomerid = 11242
Affinity DataIC50: 250nMAssay Description:Inhibition of STAT3 in human HeLa cells after 24 hrs by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Inhibition of STAT3 phosphorylation in human MDA-MB-231 cells by sandwich ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.93E+3nMAssay Description:Inhibition of human recombinant STAT3 assessed as reduction in DNA binding activity with HepG2 nuclear extract incubated for 1 hr by ELISA assayMore data for this Ligand-Target Pair